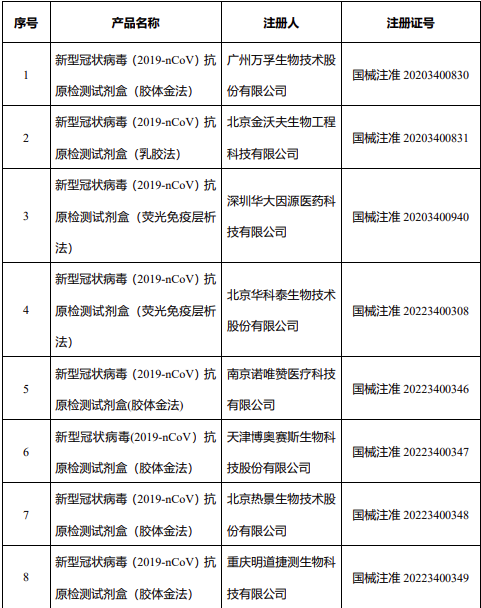
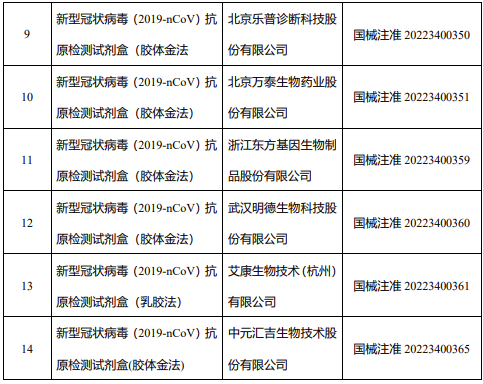
14 New Coronavirus Antigen Detection Reagents
People's Daily Online, Beijing, March 18 (Reporter Sun Hongli) According to the official website of the State Food and Drug Administration, on March 17, after review by the State Food and Drug Administration, a new coronavirus antigen detection reagent product was approved. As of March 17, the State Food and Drug Administration has approved 14 new coronavirus antigen detection reagent products.
The State Food and Drug Administration stated that the new coronavirus antigen detection reagent is suitable for the population specified in the "New Coronavirus Antigen Detection Application Program (Trial)" (Joint Prevention and Control Mechanism Zongfa [2022] No. 21). The drug supervision and administration department will strengthen post-market supervision of related products to protect the safety of patients using devices.
According to the data, on March 12, the State Food and Drug Administration issued a notice to approve the self-testing application of new coronavirus antigen products of Nanjing Novizan, Beijing Jinwofu, Shenzhen Huada Yinyuan, Guangzhou Wanfu Bio, and Beijing Huaketai Bio. change.
On March 13, the State Food and Drug Administration approved the registration applications of 5 new coronavirus antigen products, namely Wantai Bio, Rejing Bio, Tianjin Boose Bio, Chongqing Mingdao Jietest Bio, and Beijing Lepu Diagnostics.
On March 15, the State Food and Drug Administration approved the registration application of two new coronavirus antigen products, namely Zhejiang Oriental Bio and Wuhan Mingde Bio.
On March 16, the State Food and Drug Administration approved the registration application for the novel coronavirus antigen product of Aikang Biotechnology (Hangzhou) Co., Ltd.
The State Food and Drug Administration has approved the new coronavirus antigen detection reagent.
Post time: Mar-18-2022